Protactinium
91
Pa
Gruppe
I/T
Periode
7
Blokk
f
Protoner
Elektroner
Nøytroner
91
91
140
Generelle egenskaper
Atomnummer
91
Atomvekt
231,03588
Massetall
231
Kategori
Aktinoider
Farge
Sølv
Radioaktiv
Ja
From the Greek protos meaning first
Krystallstruktur
Romsentrert tetragonal
Historie
In 1900, William Crookes isolated protactinium as an intensely radioactive material from uranium
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring in Germany.
A more stable isotope of protactinium was discovered in 1917 by Otto Hahn and Lise Meitner at the Kaiser Wilhelm Institute in Berlin.
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring in Germany.
A more stable isotope of protactinium was discovered in 1917 by Otto Hahn and Lise Meitner at the Kaiser Wilhelm Institute in Berlin.
Elektroner per energinivå
2, 8, 18, 32, 20, 9, 2
Elektronkonfigurasjon
[Rn] 5f2 6d1 7s2
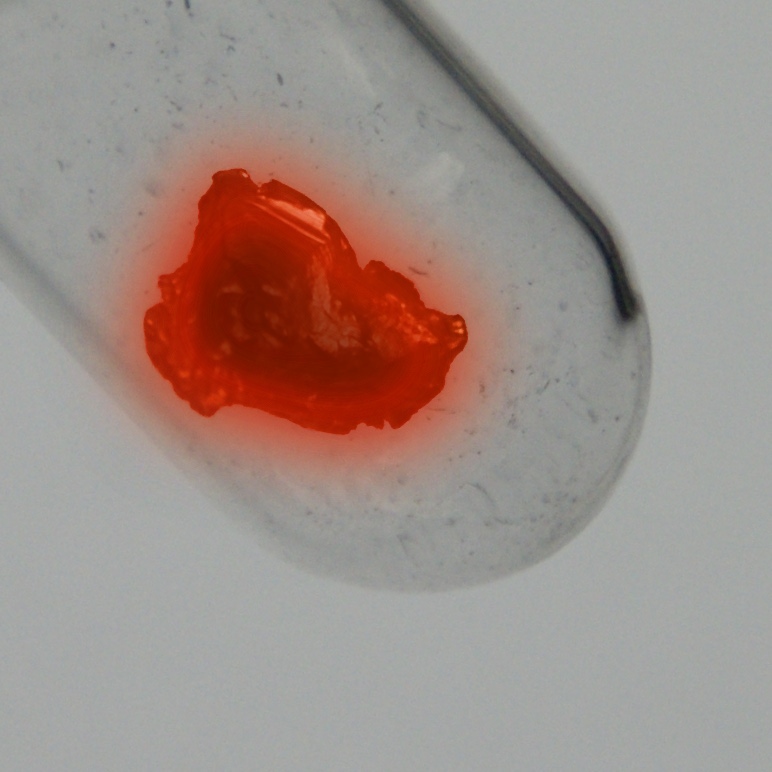
Protactinium is one of the rarest and most expensive naturally occurring elements
Fysikalske egenskaper
Fase
Fast stoff
Tetthet
15,37 g/cm3
Smeltepunkt
1841,15 K | 1568 °C | 2854,4 °F
Kokepunkt
4300,15 K | 4027 °C | 7280,6 °F
Smeltevarme
15 kJ/mol
Fordampningsvarme
470 kJ/mol
Spesifikk varmekapasitet
- J/g·K
Forekomst i jordskorpa
9,9×10-13%
Forekomst i universet
I/T
CAS-nummer
7440-13-3
PubChem Sammensatt identifikasjonsnummer (CID-nummer)
I/T
Atomegenskaper
Atomradius
163 pm
Kovalent radius
200 pm
Elektronegativitet
1,5 (Pauling-skalaen)
Ioniseringsenergi
5,89 eV
Molart volum
15,0 cm3/mol
Termisk ledningsevne
0,47 W/cm·K
Oksidasjonstilstander
3, 4, 5
Bruksområder
Owing to its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside of scientific research.
With the advent of highly sensitive mass spectrometers, an application of 231Pa as a tracer in geology and paleoceanography has become possible.
Protactinium-231 combined with the thorium-230 can be used to date marine sediments.
With the advent of highly sensitive mass spectrometers, an application of 231Pa as a tracer in geology and paleoceanography has become possible.
Protactinium-231 combined with the thorium-230 can be used to date marine sediments.
Protactinium is toxic and highly radioactive
Isotoper
Stabile isotoper
-Ustabile isotoper
212Pa, 213Pa, 214Pa, 215Pa, 216Pa, 217Pa, 218Pa, 219Pa, 220Pa, 221Pa, 222Pa, 223Pa, 224Pa, 225Pa, 226Pa, 227Pa, 228Pa, 229Pa, 230Pa, 231Pa, 232Pa, 233Pa, 234Pa, 235Pa, 236Pa, 237Pa, 238Pa, 239Pa, 240Pa